Left-Right Patterning in the Vertebrate Embryo
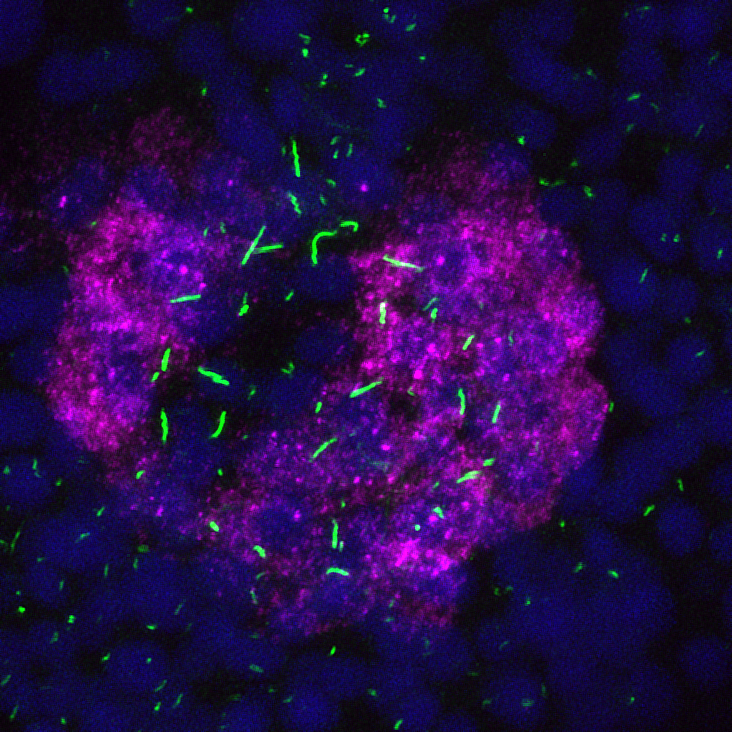
Asymmetric expression of the Nodal inhibitor dand5 (magenta) in the zebrafish ciliated (green) left-right organizer.
We use the zebrafish to study how the left-right (LR) axis is established. Vertebrates appear bilaterally symmetric, but have internal asymmetries along the LR axis, as revealed by the asymmetric placement of organs. For example, the human heart is located on the left of the body cavity, while the liver is located on the right. Defects in LR patterning can lead to birth anomalies, including congenital heart defects, that are often fatal. Current models propose cilia driven flow is required to establish LR patterning, but we still lack a full understanding of how flow is translated into a signal that is understood and responded to by relevant cells to produce asymmetric gene expression in the embryo. Without a full understanding of the genes involved and the signaling pathways utilized, we are missing important information about a process that contributes substantially to congenital heart defects in humans. Our current studies focus on identifying new players in LR patterning that will help define the pathway downstream of flow that produces asymmetric gene expression.
- Selected Publications:
- Bicc1 and Dicer regulates left-right patterning through post-transcriptional control of the Nodal inhibitor Dand5
- Gdf3 is required for robust Nodal signaling during germ layer formation and left-right patterning
- Two additional midline barriers function with midline lefty1 expression to maintain asymmetric Nodal signaling during left-right axis specification in zebrafish
Understanding how organs obtain asymmetric positions
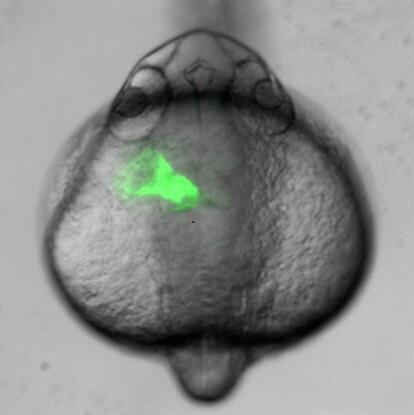
GFP expressing heart tube is asymmetrically positioned to the left of the midline.
How an organ obtains its final asymmetric position is not well understood. Organs such as the heart and pancreas form at the midline and obtain asymmetric positions later in development. Asymmetry is governed by the left-sided expression of Nodal early in development, yet each organ responds differently to this signal; for example, the heart loops to the right, while the liver is positioned on the left. To better understand how organs respond to asymmetric information, we are investigating how the developing heart responds to Nodal. Our current studies focus on implementing genomic approaches to identify Nodal-induced transcriptomic changes that drive asymmetric organ morphogenesis. Using confocal imaging, we take advantage of the transparency of zebrafish embryos to image asymmetric heart development in real time to better understand the collective cell movements that occur in this process. These studies additionally shed light on cell migration mechanisms that can be utilized by cancer cells to metastasize.
- Selected Publications:
- Cooperation between Nodal and FGF signaling regulates zebrafish cardiac cell migration and heart morphogenesis
- Left-right asymmetric heart jogging increases the robustness of dextral heart looping in zebrafish
- Integration of nodal and BMP signals in the heart requires FoxH1 to create left-right differences in cell migration rates that direct cardiac asymmetry
Utilizing Zebrafish as a Model to Study Human Disease
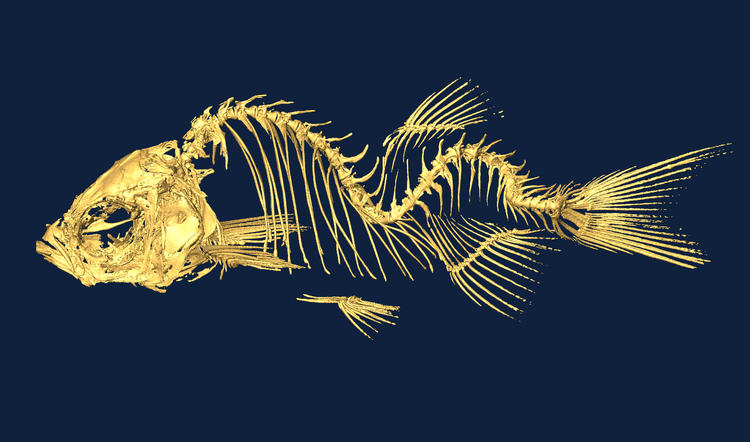
Fish lacking cilia motility develop a form of scoliosis.
Zebrafish are an excellent model for studying human disease, in part thanks to their genetic similarity to humans, their ability to be easily manipulated biologically and chemically, and their transparency which allows for feasible observation of disease progression. Variants of unknown significance from patient whole genome sequencing can often be rapidly evaluated in zebrafish providing information critical for correct diagnoses. We have developed models to study genes involved in ciliopathies, idiopathic scoliosis, and RASopathies. Our studies provide insights into disease mechanisms, can identify potential therapeutic targets, and can enable screening of drug libraries for novel drug interventions.
- Selected Publications:
- Abrogration of MAP4K4 protein function causes congenital anomalies in humans and zebrafish
- Zebrafish models of idiopathic scoliosis link cerebrospinal fluid flow defects to spine curvature
- Divergent effects of intrinsically active MEK variants on developmental Ras signaling
- In vivo severity ranking of Ras pathway mutations associated with developmental disorders
- The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation

